Lusutrombopag
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lusutrombopag is a thrombopoietin receptor agonist that is FDA approved for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. Common adverse reactions include headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Lusutrombopag is indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure.
Dosage
- Begin lusutrombopag dosing 8-14 days prior to a scheduled procedure.
- Patients should undergo their procedure 2-8 days after the last dose.
- Recommended Dosage: 3 mg orally once daily with or without food for 7 days.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding lusutrombopag Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding lusutrombopag Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding lusutrombopag Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
- There is limited information regarding lusutrombopag Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
Thrombotic/Thromboembolic Complications
- Lusutrombopag is a thrombopoietin (TPO) receptor agonist, and TPO receptor agonists have been associated with thrombotic and thromboembolic complications in patients with chronic liver disease. Portal vein thrombosis has been reported in patients with chronic liver disease treated with TPO receptor agonists. Portal vein thrombosis was reported in 1% (2 of 171) of lusutrombopag-treated patients and 1% (2 of 170) of placebo-treated patients in 3 randomized, double-blind trials and was identified post-procedure in protocol-specified imaging. The thromboses were not associated with a marked increase in platelet count.
- Consider the potential increased thrombotic risk when administering lusutrombopag to patients with known risk factors for thromboembolism, including genetic pro-thrombotic conditions (Factor V Leiden, Prothrombin 20210A, Antithrombin deficiency, or Protein C or S deficiency). In patients with ongoing or prior thrombosis or absence of hepatopetal blood flow, lusutrombopag should only be used if the potential benefit to the patient justifies the potential risk.
- Lusutrombopag should not be administered to patients with chronic liver disease in an attempt to normalize platelet counts.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of lusutrombopag was evaluated in 3 randomized, double-blind, placebo-controlled trials, L-PLUS 1, L-PLUS 2, and M0626, in which patients with chronic liver disease and thrombocytopenia were treated with lusutrombopag (N=171) or placebo (N=170) at a dose of 3 mg daily for up to 7 days prior to a scheduled procedure.
- The majority of patients were males (59%), and median age was 61 years (range 19-88). The racial and ethnic distribution was White (50%), Asian (47%), Black (<1%), and Other (3%).
- The most common adverse reactions (those occurring in at least 3%) in the lusutrombopag-treated group across the pooled data from the three trials are summarized in table 1.
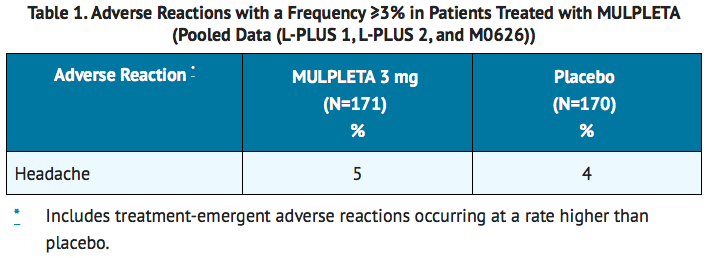
- The incidence of serious adverse events was 5% (9 of 171 patients) in the lusutrombopag group and 7% (12 of 170 patients) in the placebo group. The most common serious adverse reaction reported with lusutrombopag was portal vein thrombosis. No adverse reactions resulted in discontinuation of lusutrombopag.
Postmarketing Experience
There is limited information regarding Lusutrombopag Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Lusutrombopag Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There are no available data on lusutrombopag in pregnant women to inform the drug-associated risk. In animal reproduction studies, oral administration of lusutrombopag to pregnant rats during organogenesis and the lactation period resulted in adverse developmental outcomes. These findings were observed at exposures based on AUC that were substantially higher than the AUC observed in patients (approximately 89 times) at the recommended clinical dose of 3 mg once daily. Advise pregnant women of the potential risk to a fetus.
- The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the general population in the United States, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
- In an embryo-fetal development study in rats, lusutrombopag was orally administered during organogenesis at doses of 4, 12.5, 40, and 80 mg/kg/day. Low body weight and a decrease in the number of ossified sternebrae were noted in fetuses at 80 mg/kg/day (approximately 251 times the AUC observed in patients at the recommended clinical dose of 3 mg once daily). Minor skeletal variations (supernumerary ribs) were observed at doses of 4 mg/kg/day (approximately 23 times the AUC observed in patients at the recommended clinical dose of 3 mg once daily).
- In an embryo-fetal development study in rabbits following oral administration of lusutrombopag at doses up to 1000 mg/kg/day, no effect of lusutrombopag was observed on any parameter of embryo-fetal development.
- In a pre- and postnatal development study in rats at oral doses of 1, 4, 12.5, and 40 mg/kg/day, there were adverse effects of lusutrombopag on postnatal development at 40 mg/kg/day (approximately 230 times the AUC observed in patients at the recommended clinical dose of 3 mg once daily). The effects included prolongation of the gestation period in dams, low viability before weaning, delayed postnatal growth (delayed negative geotaxis, delayed eyelid opening, or low pup body weight), abnormal clinical signs (prominent annular rings on the tail after weaning), low fertility index, a low number of corpora lutea or implantations, and increased pre-implantation loss. The incidence of short thoracolumbar supernumerary ribs on postnatal Day 4 of F1 pups was high at doses of 12.5 mg/kg/day or more (approximately 89 times the AUC observed in patients at the recommended clinical dose of 3 mg once daily).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lusutrombopag in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Lusutrombopag during labor and delivery.
Nursing Mothers
Risk Summary
- There is no information regarding the presence of lusutrombopag in human milk, the effects on the breastfed child, and the effects on milk production. Lusutrombopag was present in the milk of lactating rats. Due to the potential for serious adverse reactions in a breastfed child, breastfeeding is not recommended during treatment with lusutrombopag and for at least 28 days after the last dose.
Clinical Considerations
Minimizing Exposure
- A lactating woman should interrupt breastfeeding and pump and discard breast milk during lusutrombopag treatment and for 28 days after the last dose of lusutrombopag in order to minimize exposure to a breastfed child.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Clinical studies of lusutrombopag did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Gender
There is no FDA guidance on the use of Lusutrombopag with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lusutrombopag with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Lusutrombopag in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Lusutrombopag in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Lusutrombopag in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Lusutrombopag in patients who are immunocompromised.
Administration and Monitoring
Administration
Recommended Dosage
- Begin lusutrombopag dosing 8-14 days prior to a scheduled procedure.
- Patients should undergo their procedure 2-8 days after the last dose.
- The recommended dosage of lusutrombopag is 3 mg taken orally once daily with or without food for 7 days. In the case of a missed dose of lusutrombopag, patients should take the missed dose as soon as possible on the same day and return to the normal schedule the following day.
- Lusutrombopag has been investigated only as a single 7-day once daily dosing regimen in clinical trials in patients with chronic liver disease. Lusutrombopag should not be administered to patients with chronic liver disease in an attempt to normalize platelet counts.
Monitoring
- Obtain a platelet count prior to initiation of lusutrombopag therapy and not more than 2 days before the procedure.
IV Compatibility
There is limited information regarding the compatibility of Lusutrombopag and IV administrations.
Overdosage
- No antidote for lusutrombopag overdose is known.
- In the event of overdose, platelet count may increase excessively and result in thrombotic or thromboembolic complications. Closely monitor the patient and platelet count. Treat thrombotic complications in accordance with standard of care.
- Hemodialysis is not expected to enhance the elimination of lusutrombopag because lusutrombopag is highly bound to protein in plasma.
Pharmacology
| |
Lusutrombopag
| |
| Systematic (IUPAC) name | |
| (E)-3-[2,6-Dichloro-4-[[4-[3-[(1S)-1-hexoxyethyl]-2-methoxyphenyl]-1,3-thiazol-2-yl]carbamoyl]phenyl]-2-methylprop-2-enoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | B02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- Lusutrombopag is an orally bioavailable, small molecule TPO receptor agonist that interacts with the transmembrane domain of human TPO receptors expressed on megakaryocytes to induce the proliferation and differentiation of megakaryocytic progenitor cells from hematopoietic stem cells and megakaryocyte maturation.
Structure
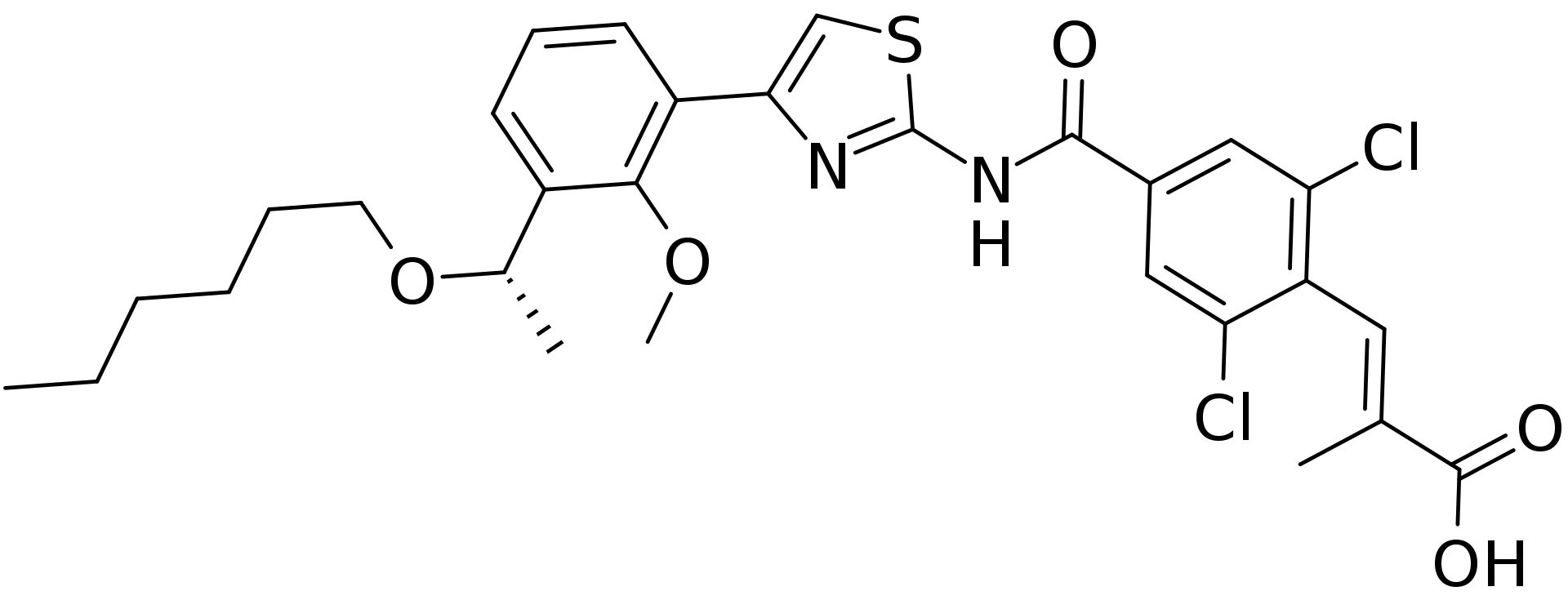
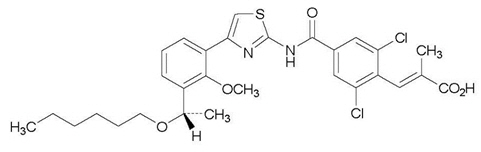
- The empirical formula for lusutrombopag is C29H32Cl2N2O5S and the molecular weight is 591.54.
- The structural formula is:
Pharmacodynamics
Platelet Response
- Lusutrombopag upregulates the production of platelets through its agonistic effect on human TPO receptors.
- The effect of lusutrombopag on platelet count increase was correlated with the AUC across the studied dose range of 0.25 mg to 4 mg in thrombocytopenic patients with chronic liver disease. With the 3 mg daily dose, the mean (standard deviation) maximum platelet count in patients (N=74) without platelet transfusion was 86.9 (27.2) × 10 9/L, and the median time to reach the maximum platelet count was 12.0 (5 to 35) days.
Cardiac Electrophysiology
- At a dose 8 times the recommended dosage, lusutrombopag does not prolong QT interval to any clinically relevant extent.
Pharmacokinetics
- Lusutrombopag demonstrated dose-proportional pharmacokinetics after single doses ranging from 1 mg (0.33 times the lowest approved dosage) to 50 mg (16.7 times the highest recommended dosage). Healthy subjects administered 3 mg of lusutrombopag had a geometric mean (%CV) maximal concentration (C max) of 111 (20.4) ng/mL and area under the time-concentration curve extrapolated to infinity (AUC 0-inf) of 2931 (23.4) ng.hr/mL. The pharmacokinetics of lusutrombopag were similar in both healthy subjects and the chronic liver disease population.
- The accumulation ratios of C max and AUC were approximately 2 with once-daily multiple-dose administration, and steady-state plasma lusutrombopag concentrations were achieved after Day 5.
Absorption
- In patients with chronic liver disease, the time to peak lusutrombopag concentration (T max) was observed 6 to 8 hours after oral administration.
Food Effect
- Lusutrombopag AUC and C max were not affected when lusutrombopag was co-administered with a high-fat meal (a total of approximately 900 calories, with 500, 250, and 150 calories from fat, carbohydrate, and protein, respectively).
Distribution
- The mean (%CV) lusutrombopag apparent volume of distribution in healthy adult subjects was 39.5 (23.5) L. The plasma protein binding of lusutrombopag is more than 99.9%.
Elimination
- The terminal elimination half-life (t 1/2) in healthy adult subjects was approximately 27 hours. The mean (%CV) clearance of lusutrombopag in patients with chronic liver disease is estimated to be 1.1 (36.1) L/hr.
Metabolism
- Lusutrombopag is primarily metabolized by CYP4 enzymes, including CYP4A11.
Excretion
- Fecal excretion accounted for 83% of the administered dose, with 16% of the dose excreted as unchanged lusutrombopag, and urinary excretion accounted for approximately 1%.
Specific Populations
- No clinically significant differences in the pharmacokinetics of lusutrombopag were observed based on age or race/ethnicity. Though lusutrombopag exposure tends to decrease with increasing body weight, differences in exposure are not considered clinically relevant.
Patients with Renal Impairment
- A population pharmacokinetic analysis did not find a clinically meaningful effect of mild (creatinine clearance (CLcr) 60 to less than 90 mL/min) and moderate (CLcr 30 to less than 60 mL/min) renal impairment on the pharmacokinetics of lusutrombopag. Data in patients with severe renal impairment (CLcr less than 30 mL/min) are limited.
Patients with Hepatic Impairment
- No clinically significant differences in the pharmacokinetics of lusutrombopag were observed based on mild to moderate (Child-Pugh class A and B) hepatic impairment.
- The mean observed lusutrombopag C max and AUC 0-τ decreased by 20% to 30% in patients (N=5) with severe (Child-Pugh class C) hepatic impairment compared to patients with Child-Pugh class A and class B liver disease. However, the ranges for C max and AUC 0-τ overlapped among patients with Child-Pugh class A, B, and C liver disease.
Drug Interaction Studies
Clinical Studies
- No clinically significant changes in lusutrombopag exposure were observed when co-administered with cyclosporine (an inhibitor of P-gp and BCRP) or an antacid containing a multivalent cation (calcium carbonate).
- No clinically significant changes in midazolam (a CYP3A substrate) exposure were observed when co-administered with lusutrombopag.
In Vitro Studies
- CYP Enzymes: lusutrombopag has low potential to inhibit CYP enzymes (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5). Lusutrombopag did not induce CYP1A2, CYP2C9, or CYP3A4.
- UGT Enzymes: lusutrombopag did not induce UGT1A2, UGT1A6, or UGT2B7.
- Transporter Systems: lusutrombopag is a substrate of P-gp and BCRP. Lusutrombopag has low potential to inhibit P-gp, BCRP, OATP1B1, OATP1B3, OCT1, OCT2, OAT1, OAT3, MATE1, MATE2-K, and BSEP.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In 2-year studies, lusutrombopag was not carcinogenic to rats at oral doses up to 20 mg/kg/day in males and 2 mg/kg/day in females (a dose 49 times and 30 times, respectively, the human exposure (AUC) at the recommended clinical dose of 3 mg/day for 7 days) and to mice at oral doses up to 20 mg/kg/day in males and females (a dose approximately 45 times the human exposure (AUC) at the recommended clinical dose of 3 mg/day for 7 days).
- Lusutrombopag was not genotoxic based on an in vitro bacterial reverse mutation (Ames) assay, a chromosomal aberration assay with cultured Chinese hamster lung cells, and an in vivo micronucleus assay with mouse bone marrow cells.
- In a fertility and early embryonic development study, lusutrombopag did not affect fertility in male and female rats at oral doses up to 100 mg/kg/day (a dose in males and females approximately 176 and 252 times, respectively, the human exposure (AUC) at the recommended clinical dose of 3 mg/day for 7 days).
Clinical Studies
- The efficacy of lusutrombopag for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo a procedure was evaluated in 2 randomized, double-blind, placebo-controlled trials (L-PLUS 1 (N=97) and L-PLUS 2 (N=215; NCT02389621)). Patients with chronic liver disease who were undergoing an invasive procedure and had a platelet count less than 50 × 109/L were eligible to participate. Patients undergoing laparotomy, thoracotomy, open-heart surgery, craniotomy, or organ resection were excluded. Patients with a history of splenectomy, partial splenic embolization, or thrombosis and those with Child-Pugh class C liver disease, absence of hepatopetal blood flow, or a prothrombotic condition other than chronic liver disease were not allowed to participate.
- The patient populations were similar between the lusutrombopag and placebo arms and consisted of 60% male and 40% female; median age was 60 years (range 19-88). The racial and ethnic distribution was White (55%), Asian (41%), and Other (4%).
- Patients were randomized 1:1 to receive 3 mg of lusutrombopag or placebo once daily for up to 7 days. Randomization was stratified by liver ablation/coagulation or other procedures and the platelet count at screening/baseline. In L-PLUS 1, 57% of patients underwent procedures other than liver ablation/coagulation and 43% underwent liver ablation/coagulation (RFA/MCT). In L-PLUS 2, 98% of patients underwent procedures other than liver ablation/coagulation and 2% underwent liver ablation/coagulation (RFA/MCT). Procedures other than liver ablation/coagulation (RFA/MCT) included liver-related procedures (transcatheter arterial chemoembolization, liver biopsy, and others), upper and lower gastrointestinal endoscopy-related procedures (endoscopic variceal ligation, endoscopic injection sclerotherapy, polypectomy, and biopsy), and other procedures (dental extraction, diagnostic paracentesis or laparocentesis, septoplasty, embolization of splenic artery aneurysm, bone marrow biopsy, removal of cervical polyp, and inguinal hernia repair (non-laparotomy based)).
- In L-PLUS 1, the major efficacy outcome was the proportion of patients who require no platelet transfusion prior to the primary invasive procedure. In L-PLUS 2, the major efficacy outcome was the proportion of patients who require no platelet transfusion prior to the primary invasive procedure and no rescue therapy for bleeding (i.e., platelet preparations, other blood preparations, including red blood cells and plasma, volume expanders) from randomization through 7 days after the primary invasive procedure. In both trials, additional efficacy outcomes included the proportion of patients who require no platelet transfusion during the study, proportion of responders, duration of the increase in platelet count defined as the number of days during which the platelet count was maintained as ≥50 × 10 9/L, and the time course of platelet counts.
- In both the L-PLUS 1 and L-PLUS 2 trials, responders were defined as patients who had a platelet count of ≥50 × 10 9/L with an increase of ≥20 × 10 9/L from baseline.
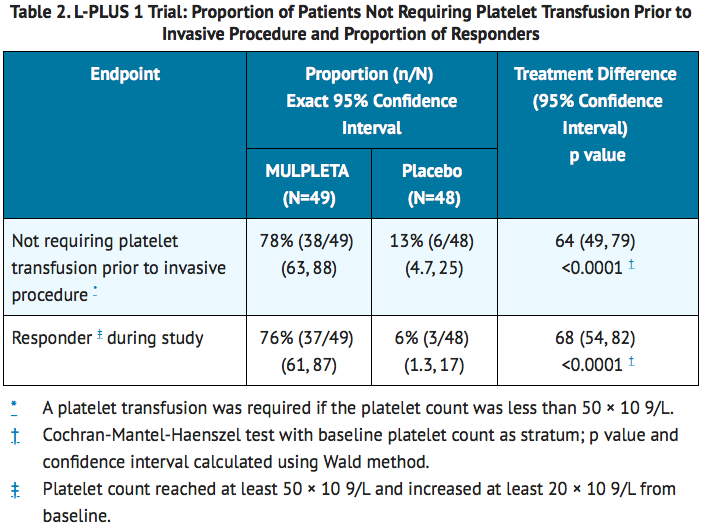

- The median (Q1, Q3) duration of platelet count increase to at least 50 × 10 9/L was 22 (17, 27) days in lusutrombopag-treated patients without platelet transfusion and 1.8 (0.0, 8.3) days in placebo-treated patients with platelet transfusion in L-PLUS 1 and 19 (13, 28) days in lusutrombopag-treated patients without platelet transfusion and 0.0 (0.0, 5.0) days in placebo-treated patients with platelet transfusion in L-PLUS 2.
How Supplied
- Lusutrombopag is supplied as 3 mg lusutrombopag tablets in a child-resistant blister pack containing 7 tablets.
Storage
- Store lusutrombopag in the original package at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Lusutrombopag |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Lusutrombopag |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Prior to treatment, patients should fully understand and be informed of the following risks and considerations for lusutrombopag.
Risks
Thrombotic/Thromboembolic Complications
- Lusutrombopag is a thrombopoietin (TPO) receptor agonist, and TPO receptor agonists have been associated with thrombotic and thromboembolic complications in patients with chronic liver disease. Portal vein thrombosis has been reported in patients with chronic liver disease treated with TPO receptor agonists.
Pregnancy
- Advise women of reproductive potential who become pregnant or are planning to become pregnant that lusutrombopag should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
Lactation
- Advise women not to breastfeed during treatment with lusutrombopag and for 28 days after the last dose of lusutrombopag. Advise women to pump and discard breast milk during this period.
Patient Package Insert
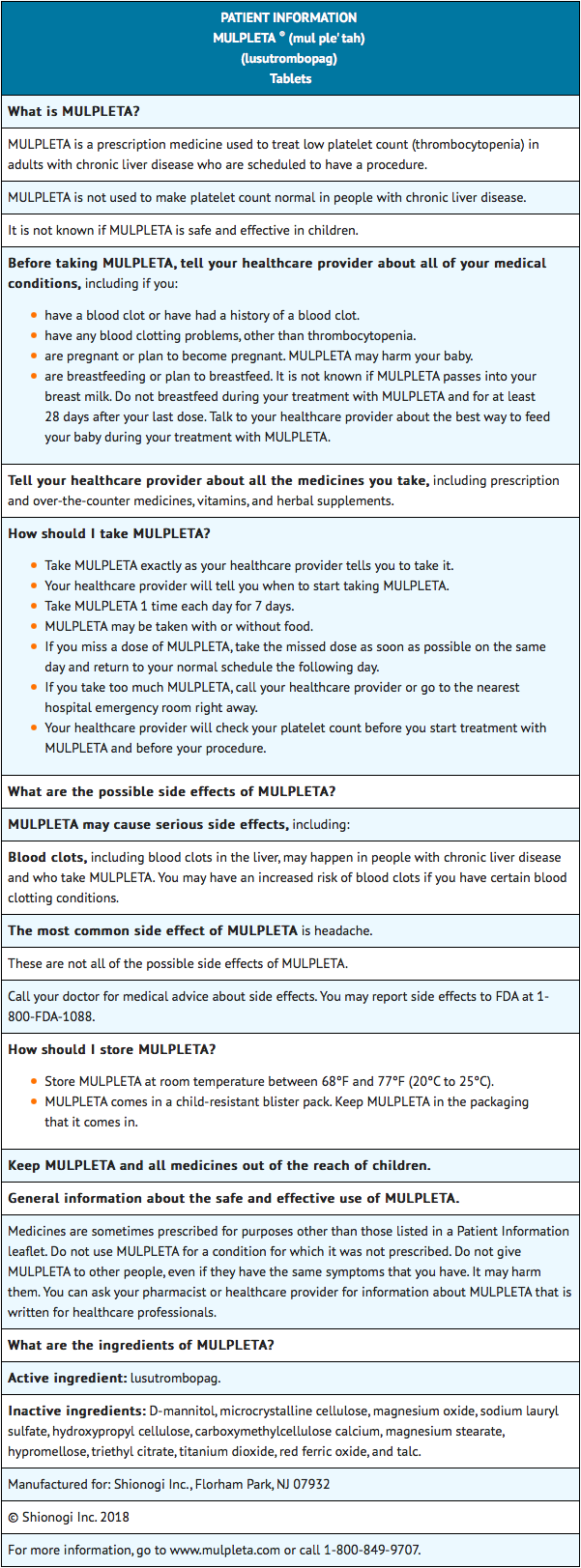
Precautions with Alcohol
Alcohol-Lusutrombopag interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Lusutrombopag Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.